Next: pH and Dissociation of
Up: Lab-3 Background
Previous: Lab-3 Background
 Solvent
Solvent
- Water is an excellent solvent, which means
that many chemicals dissolve easily into it
(referred to as dissolved species, Fig. 4.2).
 Dipolar
Dipolar
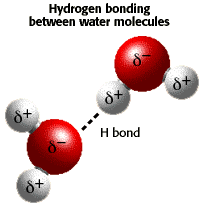
- Water molecules have one end that is more negatively
charged, and one end more positively charged
(Fig. 4.1), allowing it to bond or complex
with most charged species and with itself.
 Benefits
Benefits
- Makes water a useful cleaning agent, and important
transporter of chemicals in all living things
 Costs
Costs
- Water can easily be contaminated with undesirable
chemicals
Figure 4.1:
Dipolar
nature of water molecule and intermolecular hydrogen bond. The two
white hydrogens are more positive than the red oxygen (which is not
sharing two of its electrons). After
U. Arizona Biology Project.
|
|




Next: pH and Dissociation of
Up: Lab-3 Background
Previous: Lab-3 Background
GEOS 3110 Professor's Notes, Summer 2003
Dr. T. Brikowski, UTD