Lecture Notes from CHM 1341
18 June 1996
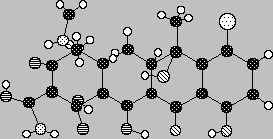 Molecular Shapes
Molecular Shapes
We're now in a position to rationalize the shapes of molecules based upon the numbers of bonding (unpaired) and non-bonding (lone paired) electrons at the atomic bonding centers. The electron clouds about and between atoms have definite geometries and interactions. These drive the geometries of molecules as evidenced by the resting position of the atomic nuclei in them.
Clicking on the biomolecule below takes you to the University of California at San Diego where Kent Wilson's group wants to explain why Quantum Mechanics is important in the study of Life.
 Molecular shape is of absolutely critical importance to Life. Our cells go to a great deal of trouble to manufacture enormous (on the molecular scale) protein molecules whose primary purpose is to conform to particular shapes. The easiest of these to explain are the enzymes, biological catalysts which enhance, retard, or eliminate chemical reactions in and around our cells. They can do so by providing niches which fit the molecule's they are to influence even better than OJ 's gloves. Their target compounds are thus locked in place awaiting action, such as the arrival of a reactant similarly drawn to its most advantageous resting place. These kinds of enzymes are matchmakers, promoting efficient body chemistry. Others retard deleterious effects, like oxidation occuring in the wrong place or at the wrong time. So we are critically beholden to molecular geometry as are all living things!
Molecular shape is of absolutely critical importance to Life. Our cells go to a great deal of trouble to manufacture enormous (on the molecular scale) protein molecules whose primary purpose is to conform to particular shapes. The easiest of these to explain are the enzymes, biological catalysts which enhance, retard, or eliminate chemical reactions in and around our cells. They can do so by providing niches which fit the molecule's they are to influence even better than OJ 's gloves. Their target compounds are thus locked in place awaiting action, such as the arrival of a reactant similarly drawn to its most advantageous resting place. These kinds of enzymes are matchmakers, promoting efficient body chemistry. Others retard deleterious effects, like oxidation occuring in the wrong place or at the wrong time. So we are critically beholden to molecular geometry as are all living things!
Valence Shell Electron Pair Repulsion (VSEPR) Theory is predicated upon the notion that within the valence shell, the electrons will stay as far from one another as is possible consistent with the Lewis notion that they must begin pairing up after Group IV. That VSEPR speaks of pairs and not individual electrons is because it, like Lewis, considers the electrons shared in bonds to be part of an atom's valence shell. So when the atom's valence has been "satisfied" via chemical bonds, there will certainly be bonding electron pairs and there will likely be non-bonding electron pairs as well. At the level of Gillespie, et al., no distinction is drawn between these two kinds of pairs.
In later courses, we'll see that all repulsions are not created equal. Lone pair/lone pair are strongest. Lone pair/bond pair repulsions are less strong. And bond pair/bond pair exhibits the weakest repulsion. All that means is that the lone pairs, uncompromised in bonds, bully the bonding pairs somewhat. This is seen as influences in bond angles mostly.
The shapes that result from electron pairs running as far from one another as possible are easy to imagine. Take Cl-Be-Cl; the central bonding atom, Be, has only 2 bonding pairs. So they will align themselves 180 degrees apart. That makes Cl-Be-Cl a linear molecule. Notice that we fixated on the Be and not the Cl; each Cl was merely the source of a (not very well shared) electron of the bonding pair.
In the parlance of VSEPR bonding this is designated as
A X2 yields linear molecular geometry
meaning that the central atom (always designated "A") has two bonding pairs of electrons (each designated as "X") and no non-bonding "lone" pairs (which would have been designated "E" had there been any).
If we move up to boron trichloride, the 3 bonding pairs will align themselves 120 degrees apart making a planar triangular molecule:
..
:Cl:
|
B designated A X3 yielding triangular planar
/ \
:Cl: :Cl:
'' ''
Again the chlorines are treated as mere (grudging) donors of an electron each to the bonding pairs. We don't mean to malign chorine thus; there are, of course, molecules in which Cl is the bonding center, but they are the subjects of more advanced courses.
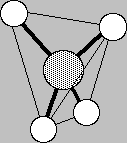 Finally, we come to the most flexible atom on the table, carbon. C can make bonds to up to 4 atoms at a time, and they can be linked into the interesting macromolecules of Life. Carbon is the architect of so much interesting geometry because its four bonds, in methane say, array themselves to the corners of a perfect tetrahedron (all the hydrogens are equidistant from carbon and from one another), the most elementary of the Platonic solids.
Finally, we come to the most flexible atom on the table, carbon. C can make bonds to up to 4 atoms at a time, and they can be linked into the interesting macromolecules of Life. Carbon is the architect of so much interesting geometry because its four bonds, in methane say, array themselves to the corners of a perfect tetrahedron (all the hydrogens are equidistant from carbon and from one another), the most elementary of the Platonic solids.
 Notice that it's not just to the 4 corners of the compass that carbon points; that would be only planar. Carbon points out into 3-dimensional space! It facilitates 3-d molecular architecture like that of diamond at left. That rigid lattice is the reason that diamond is the second hardest substance know. (The 1st is boron nitride.)
Notice that it's not just to the 4 corners of the compass that carbon points; that would be only planar. Carbon points out into 3-dimensional space! It facilitates 3-d molecular architecture like that of diamond at left. That rigid lattice is the reason that diamond is the second hardest substance know. (The 1st is boron nitride.)
It would take only a little geometry to conclude that the H-C-H angles in methane are now arc cos(-1/3) = 109.47 degrees. Thus methane's designation
A X4 yields tetrahedral geometry.
 As soon as we've passed Group IV, the valence shell electrons must start pairing up. Thus nitrogen has 1 lone pair (non-bonding) and 3 electrons to share. When it bonds, as ammonia say, there will be a total of four electron pairs again surrounding the central bonding atom (N), 3 are bonding and one non-bonding. Again, the furthest they can flee from one another is into a tetrahedral electronic configuration. However, the molecular geometry applies only to those electron pairs which are bonded. Hence, the hydrogens sit in a triangle off to one side of the nitrogen while the lone pair (not counted in molecular geometry) sticks off in the opposite direction. Thus ammonia's VSEPR designation
As soon as we've passed Group IV, the valence shell electrons must start pairing up. Thus nitrogen has 1 lone pair (non-bonding) and 3 electrons to share. When it bonds, as ammonia say, there will be a total of four electron pairs again surrounding the central bonding atom (N), 3 are bonding and one non-bonding. Again, the furthest they can flee from one another is into a tetrahedral electronic configuration. However, the molecular geometry applies only to those electron pairs which are bonded. Hence, the hydrogens sit in a triangle off to one side of the nitrogen while the lone pair (not counted in molecular geometry) sticks off in the opposite direction. Thus ammonia's VSEPR designation
A X3 E yields triangular PYRAMIDAL bonding.
 With two non-bonding pairs and two bonding pairs, the tetrahedral electronic domains give the water molecule a bent shape. Hence
With two non-bonding pairs and two bonding pairs, the tetrahedral electronic domains give the water molecule a bent shape. Hence
A X2 E2 yields bent bonding.
Finally, both ends of the period show molecules (LiH and FH, say) with linear molecular shapes since any two points (the diatomic nuclei) define a straight line. However, the two molecules have different VSEPR designations.
..
Li : H is A X1 but :F : H is A X1 E3
¨
Nevertheless, even though fluorine has a tetrahedral electronic arrangement of its electron pairs, it is a linear molecule because only the bond geometry counts.
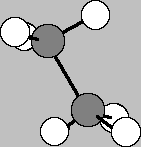 So now we can predict the shapes of polyatomic molecules that have single bonds. This molecule of ethane with its carbon-carbon bond and 3 hydrogens satisfying the remaining valence on each carbon, must exhibit tetrahedral bonding at both bonding centers. The hydrogen "umbrellas" point away from the C-C bonding axis, and, as it turns out, are relatively free to spin 'round that axis. It takes only a little imagination to replace one of the hydrogens with yet another carbon attached to yet another carbon attached to yet another carbon etc. 'til the cows come home. In so doing, we're building gasoline and oils.
So now we can predict the shapes of polyatomic molecules that have single bonds. This molecule of ethane with its carbon-carbon bond and 3 hydrogens satisfying the remaining valence on each carbon, must exhibit tetrahedral bonding at both bonding centers. The hydrogen "umbrellas" point away from the C-C bonding axis, and, as it turns out, are relatively free to spin 'round that axis. It takes only a little imagination to replace one of the hydrogens with yet another carbon attached to yet another carbon attached to yet another carbon etc. 'til the cows come home. In so doing, we're building gasoline and oils.
It's not such a leap to double and triple bonded centers as one might suppose. If you picture those unpaired electrons stuck off in tetrahedral directions, simply imagine bringing 2 tetrahedra together along a mutual edge.
 What you've constructed mentally ought to look something like ethylene there at the right. As a bonus, we note that the alignment of that tetrahedral edge casts each pair of CH bonds away from the C=C bond and renders all 6 atoms in a single plane. While rotation about single bonds is possible, rotation about multiple bonds is not. Thus, ethylene is planar. It's VSEPR designation at each C bonding center is
What you've constructed mentally ought to look something like ethylene there at the right. As a bonus, we note that the alignment of that tetrahedral edge casts each pair of CH bonds away from the C=C bond and renders all 6 atoms in a single plane. While rotation about single bonds is possible, rotation about multiple bonds is not. Thus, ethylene is planar. It's VSEPR designation at each C bonding center is
A X3 yielding planar triangular bonding at both carbons.
But wait, there's more!
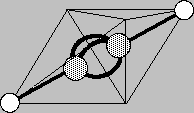 Carbon can triple bond to another leaving only one hydrogen on each center to satisfy the valence. It's common name is acetylene, or in strict chemical parlance, "ethyne" (rhymes with "wine"), and you've seen it used in welding torches. Its high carbon to hydrogen ratio makes it smoke badly (with pure carbon tendriles) if not enough oxygen is used in the torch. But our interest in it is that its triple bond results from an imaginary juxtaposition of not an electronic tetrahedral edge but a tetrahedral face (see the light lines at left). That aims three bonding electrons on each carbon at their mates from the other carbon. The triple bond which forms can be imagined to be rather voluminous since each bond appears bent. The appearance is valid even though the explanation needs massaging in later courses.
Carbon can triple bond to another leaving only one hydrogen on each center to satisfy the valence. It's common name is acetylene, or in strict chemical parlance, "ethyne" (rhymes with "wine"), and you've seen it used in welding torches. Its high carbon to hydrogen ratio makes it smoke badly (with pure carbon tendriles) if not enough oxygen is used in the torch. But our interest in it is that its triple bond results from an imaginary juxtaposition of not an electronic tetrahedral edge but a tetrahedral face (see the light lines at left). That aims three bonding electrons on each carbon at their mates from the other carbon. The triple bond which forms can be imagined to be rather voluminous since each bond appears bent. The appearance is valid even though the explanation needs massaging in later courses.
What we can say without fear of contradiction is that the coincidence of those tetrahedral faces means that their opposite vertices (the hydrogens) lie in a line with the carbon-carbon triple bond; in other words, this
A X2 molecule is linear.
Return to the CHM 1341 Lecture Notes or Go To Next or Previous Lectures.
Chris Parr
University of Texas at Dallas
Programs in Chemistry, Room BE3.506
P.O. Box 830688 M/S BE2.6 (for snailmail)
Richardson, TX 75083-0688
Voice: (214) 883-2485
Fax: (214) 883-2925
BBS: (214) 883-2168 (HST) or -2932 (V.32bis)
Internet: parr@utdallas.edu (Click on that address to send Chris e-mail.)
Last modified 17 June 1996.
 Molecular Shapes
Molecular Shapes Molecular Shapes
Molecular Shapes Molecular shape is of absolutely critical importance to Life. Our cells go to a great deal of trouble to manufacture enormous (on the molecular scale) protein molecules whose primary purpose is to conform to particular shapes. The easiest of these to explain are the enzymes, biological catalysts which enhance, retard, or eliminate chemical reactions in and around our cells. They can do so by providing niches which fit the molecule's they are to influence even better than OJ 's gloves. Their target compounds are thus locked in place awaiting action, such as the arrival of a reactant similarly drawn to its most advantageous resting place. These kinds of enzymes are matchmakers, promoting efficient body chemistry. Others retard deleterious effects, like oxidation occuring in the wrong place or at the wrong time. So we are critically beholden to molecular geometry as are all living things!
Molecular shape is of absolutely critical importance to Life. Our cells go to a great deal of trouble to manufacture enormous (on the molecular scale) protein molecules whose primary purpose is to conform to particular shapes. The easiest of these to explain are the enzymes, biological catalysts which enhance, retard, or eliminate chemical reactions in and around our cells. They can do so by providing niches which fit the molecule's they are to influence even better than OJ 's gloves. Their target compounds are thus locked in place awaiting action, such as the arrival of a reactant similarly drawn to its most advantageous resting place. These kinds of enzymes are matchmakers, promoting efficient body chemistry. Others retard deleterious effects, like oxidation occuring in the wrong place or at the wrong time. So we are critically beholden to molecular geometry as are all living things! Finally, we come to the most flexible atom on the table, carbon. C can make bonds to up to 4 atoms at a time, and they can be linked into the interesting macromolecules of Life. Carbon is the architect of so much interesting geometry because its four bonds, in methane say, array themselves to the corners of a perfect tetrahedron (all the hydrogens are equidistant from carbon and from one another), the most elementary of the Platonic solids.
Finally, we come to the most flexible atom on the table, carbon. C can make bonds to up to 4 atoms at a time, and they can be linked into the interesting macromolecules of Life. Carbon is the architect of so much interesting geometry because its four bonds, in methane say, array themselves to the corners of a perfect tetrahedron (all the hydrogens are equidistant from carbon and from one another), the most elementary of the Platonic solids. Notice that it's not just to the 4 corners of the compass that carbon points; that would be only planar. Carbon points out into 3-dimensional space! It facilitates 3-d molecular architecture like that of diamond at left. That rigid lattice is the reason that diamond is the second hardest substance know. (The 1st is boron nitride.)
Notice that it's not just to the 4 corners of the compass that carbon points; that would be only planar. Carbon points out into 3-dimensional space! It facilitates 3-d molecular architecture like that of diamond at left. That rigid lattice is the reason that diamond is the second hardest substance know. (The 1st is boron nitride.) As soon as we've passed Group IV, the valence shell electrons must start pairing up. Thus nitrogen has 1 lone pair (non-bonding) and 3 electrons to share. When it bonds, as ammonia say, there will be a total of four electron pairs again surrounding the central bonding atom (N), 3 are bonding and one non-bonding. Again, the furthest they can flee from one another is into a tetrahedral electronic configuration. However, the molecular geometry applies only to those electron pairs which are bonded. Hence, the hydrogens sit in a triangle off to one side of the nitrogen while the lone pair (not counted in molecular geometry) sticks off in the opposite direction. Thus ammonia's VSEPR designation
As soon as we've passed Group IV, the valence shell electrons must start pairing up. Thus nitrogen has 1 lone pair (non-bonding) and 3 electrons to share. When it bonds, as ammonia say, there will be a total of four electron pairs again surrounding the central bonding atom (N), 3 are bonding and one non-bonding. Again, the furthest they can flee from one another is into a tetrahedral electronic configuration. However, the molecular geometry applies only to those electron pairs which are bonded. Hence, the hydrogens sit in a triangle off to one side of the nitrogen while the lone pair (not counted in molecular geometry) sticks off in the opposite direction. Thus ammonia's VSEPR designation With two non-bonding pairs and two bonding pairs, the tetrahedral electronic domains give the water molecule a bent shape. Hence
With two non-bonding pairs and two bonding pairs, the tetrahedral electronic domains give the water molecule a bent shape. Hence So now we can predict the shapes of polyatomic molecules that have single bonds. This molecule of ethane with its carbon-carbon bond and 3 hydrogens satisfying the remaining valence on each carbon, must exhibit tetrahedral bonding at both bonding centers. The hydrogen "umbrellas" point away from the C-C bonding axis, and, as it turns out, are relatively free to spin 'round that axis. It takes only a little imagination to replace one of the hydrogens with yet another carbon attached to yet another carbon attached to yet another carbon etc. 'til the cows come home. In so doing, we're building gasoline and oils.
So now we can predict the shapes of polyatomic molecules that have single bonds. This molecule of ethane with its carbon-carbon bond and 3 hydrogens satisfying the remaining valence on each carbon, must exhibit tetrahedral bonding at both bonding centers. The hydrogen "umbrellas" point away from the C-C bonding axis, and, as it turns out, are relatively free to spin 'round that axis. It takes only a little imagination to replace one of the hydrogens with yet another carbon attached to yet another carbon attached to yet another carbon etc. 'til the cows come home. In so doing, we're building gasoline and oils. What you've constructed mentally ought to look something like ethylene there at the right. As a bonus, we note that the alignment of that tetrahedral edge casts each pair of CH bonds away from the C=C bond and renders all 6 atoms in a single plane. While rotation about single bonds is possible, rotation about multiple bonds is not. Thus, ethylene is planar. It's VSEPR designation at each C bonding center is
What you've constructed mentally ought to look something like ethylene there at the right. As a bonus, we note that the alignment of that tetrahedral edge casts each pair of CH bonds away from the C=C bond and renders all 6 atoms in a single plane. While rotation about single bonds is possible, rotation about multiple bonds is not. Thus, ethylene is planar. It's VSEPR designation at each C bonding center is Carbon can triple bond to another leaving only one hydrogen on each center to satisfy the valence. It's common name is acetylene, or in strict chemical parlance, "ethyne" (rhymes with "wine"), and you've seen it used in welding torches. Its high carbon to hydrogen ratio makes it smoke badly (with pure carbon tendriles) if not enough oxygen is used in the torch. But our interest in it is that its triple bond results from an imaginary juxtaposition of not an electronic tetrahedral edge but a tetrahedral face (see the light lines at left). That aims three bonding electrons on each carbon at their mates from the other carbon. The triple bond which forms can be imagined to be rather voluminous since each bond appears bent. The appearance is valid even though the explanation needs massaging in later courses.
Carbon can triple bond to another leaving only one hydrogen on each center to satisfy the valence. It's common name is acetylene, or in strict chemical parlance, "ethyne" (rhymes with "wine"), and you've seen it used in welding torches. Its high carbon to hydrogen ratio makes it smoke badly (with pure carbon tendriles) if not enough oxygen is used in the torch. But our interest in it is that its triple bond results from an imaginary juxtaposition of not an electronic tetrahedral edge but a tetrahedral face (see the light lines at left). That aims three bonding electrons on each carbon at their mates from the other carbon. The triple bond which forms can be imagined to be rather voluminous since each bond appears bent. The appearance is valid even though the explanation needs massaging in later courses.